全國第一 國研院儀科中心攜手台灣微創 中醫大新竹附醫全國首例國產品椎體重建手術成功
中華民國 108 年 6 月 17 日新聞資料
根據全民健保資料,在台灣每4名65歲以上民眾中,就有1人因為骨質疏鬆症造成脊椎壓迫性骨折。「椎體重建手術」是利用「椎體撐開器」將脊椎壓迫性骨折患者以垂直方式撐開塌陷的椎體,灌入骨水泥固定,達到脊椎復位、緩解長期疼痛的效果。「椎體撐開器」是技術門檻非常高的永久性植入物醫療器材,需要經過多道精密金屬加工程序,且需一體成形,品質要求非常高。國家實驗研究院台灣儀器科技研究中心輔導新創公司台灣微創開發出「椎體撐開器」(衛生福利部醫療器材許可證編號:衛部醫器製字第006305號),中國醫藥大學新竹附設醫院率先採用,領先全台完成第一例國產品椎體重建手術治療,病患於術後復原狀況良好,且於隔天離院後就可以行動自如。
台灣自主研發製造+國研院儀科中心輔導協助
隨著高齡化社會來臨,民眾的骨科意識也提高。根據全球市場研究機構TrendForce及Research and Markets調查報告顯示,2018年全球醫療器材市場規模估達4,442億美元,2023年上看5,776億美元;其中全球椎體重建手術市場規模在2026年預估達48億美元,此類手術以年成長率8.25%持續攀升,是所有脊椎手術領域中成長最為顯著、發展性非常高的產業。
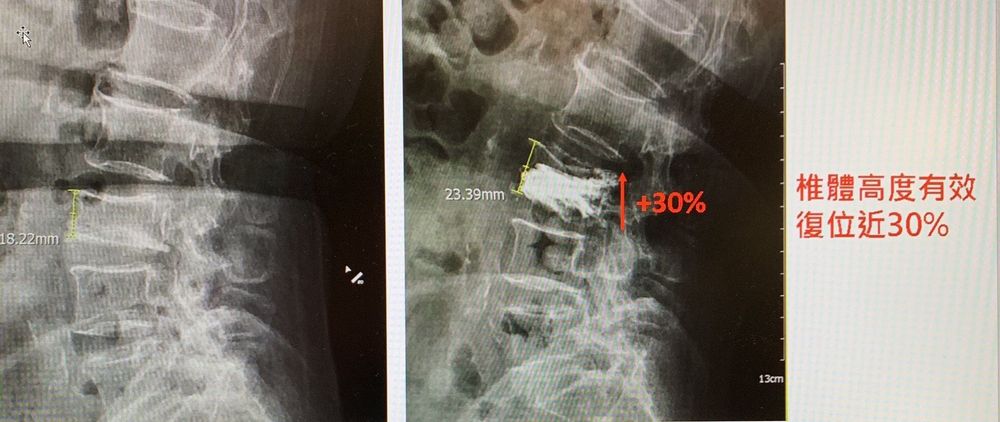
目前市面上的椎體撐開器均為國外進口廠牌,使用的方式像是利用汽車千斤頂的機械撐開原理,將千斤頂置入椎體後,再以垂直方式撐開塌陷的椎體,並灌入骨水泥固定,達到脊椎復位、緩解長期疼痛的效果。
台灣微創公司梁晃千董事長指出,和其他國外廠牌相比,台灣微創的椎體撐開器成功運用多面向推進力量,首創以3D立體支撐方式,使病患有更好的復位效果,減少疼痛不適;同時也是市面上唯一加入回退設計的安全機制,能有效預防手術中的風險產生,提高在全球市場的競爭優勢。
台灣微創是100%自主研發,且在台灣生產製造,因此更能確保產品品質。但除了技術與品質外,梁晃千董事長表示,更重要的是「透過國研院儀科中心醫材加速器的輔導,讓產品承擔的風險大大降低,創新的醫療器材產品才得以問世。」本次發表的椎體撐開器,目標是在三年內創造超過新台幣5億元的產值,讓國內外醫療院所都有機會使用到台灣的高品質創新醫材產品,造福更多病患。
透過國產品椎體撐開器有效復位改善X片
為病患醫療設想更多 中醫大新竹附醫全國首例率先採用創新醫材
80歲的劉姓婦人今年四月底在家跌倒走路跌倒,因嚴重背痛急診就醫,經中醫大新竹附醫X光發現腰椎第三節有壓迫性骨折,經進一步的核磁共振檢查後確認需安排手術治治療,為提供較好的椎體塌陷改善效果,決定採用新式設計具有多面向推進力量的3D立體支撐支架、且具有椎體塌陷復位設計之國產品來進行手術。神經外科主任黃祥銘醫師表示,劉姓病患於5月8日術後復原良好,且隔天離院後行動自如,目前狀況恢復良好背部疼痛大幅改善。
中國醫藥大學新竹附設醫院陳自諒院長指出,將貼心醫療服務與優質醫療資源帶進新竹是中醫大新竹附醫成立的初衷,為了提供病患友善的治療環境,在硬體環境與手術評估,中醫大新竹附醫更是秉持「以病人為尊、以醫院為榮」的嚴謹審慎的態度,讓患者可以獲得最高品質的醫療服務,此外,也樂見與國研院儀科中心共同攜手,為建立創新的醫療特色而努力。
中醫大新竹附醫與國研院期盼未來能有更多廠商能透過產研醫合作模式,讓創新產品可以與國研院儀科中心醫材加速器鏈結,加速商品化的步伐,使臨床需求得到更好的照護品質,也將台灣醫材品牌推向世界舞台。
法人產業醫師通力合作 催生台灣在地的高階醫材品牌
由於椎體撐開器具有高技術含量,目前市面上主要是歐美品牌,台灣微創透過國研院儀科中心及中醫大新竹附醫的協助,成為第一家通過台灣TFDA許可上市的醫療器材廠商。作為一家新創公司,台灣微創在「國研院儀科中心醫材加速器」輔導協助下,獲得原型製作、手術器械測試、手術模擬及醫材造影的技術能量,進而一路過關通過審核,而能與國外廠牌競逐國際市場,因此今(6/17)日特地在中醫大新竹附醫舉辦「台灣首例國產品椎體重建手術成功傳承儀式」,一方面見證中醫大新竹附醫卓越的醫療服務品質,一方面也向各界展示國研院儀科中心醫材加速器對台灣醫材產業的具體貢獻,藉由與中醫大新竹附醫的聯盟合作,打造新竹生醫產業聚落的蓬勃發展。
科技部謝達斌政務次長在「台灣首例國產品椎體重建手術成功傳承儀式」致詞時表示,生技醫療產業是政府重點發展的產業之一,科技部對於台灣新創的能量更是深具信心,也積極協助台灣新創團隊鏈結國內外資源,進駐新竹生醫園區台灣微創就是其中一例。科技部希望強化醫材產業群聚效應,透過台灣新創生態系的互相鏈結,加速研發成果商品化。
國研院吳光鐘副院長指出,國研院建構完整的生醫研發技術服務平台,有系統地鏈結新創能量與產業接軌,以強化台灣生醫新創產業發展,加速挺進國際市場。國研院儀科中心楊燿州主任則強調,儀科中心擁有獨一無二協助新創團隊的秘密利器「一站式醫材加速器」,專人、專案、專業的輔導協助下,幫助醫療器材加值創新,更重要的是可以大幅降低醫材產品的研發成本及風險,進而提高成功率。
新竹縣政府衛生局殷東成局長表示,生醫科技是新竹縣政府文化科技智慧城發展藍圖的重要拼圖,也是再創新竹縣30年新榮景的關鍵目標。新竹生物醫學園區及中醫大新竹附醫都落腳在新竹縣竹北市,更是造福新竹縣民。相信未來經由科技部、國研院、廠商及新竹縣政府的努力,定能形成生醫產業聚落效應,創造經濟效益,帶動新竹縣的蓬勃發展。
全國首例國產品椎體重建手術成功傳承儀式-科技部謝達斌次長(中)、中醫大新竹附醫陳自諒院長(左五)、國研院吳光鐘副院長(右五)、新竹科學園區管理局許增如副局長(左四)、新竹縣衛生局殷東成局長(右四)、中醫大新竹附醫陳俊賢副院長(左三)、國研院儀科中心楊燿州主任(右三)、中醫大新竹附醫神經外科黃祥銘主任(左二)、台灣微創公司梁晃千董事長、新竹縣議會蔡志環議員(右一)、新竹縣議會吳旭智議員(左一)
全國首例國產品椎體撐開器產研醫功臣:左起中醫大新竹附醫神經外科黃祥銘主任、國研院儀科中心楊燿州主任、中醫大新竹附醫陳俊賢副院長、台微醫梁晃千董事長
%E3%80%81%E4%B8%AD%E9%86%AB%E5%A4%A7%E6%96%B0%E7%AB%B9%E9%99%84%E9%86%AB%E9%99%B3%E8%87%AA%E8%AB%92%E9%99%A2%E9%95%B7(%E5%B7%A6%E4%BA%94)%E3%80%81%E5%9C%8B%E7%A0%94%E9%99%A2%E5%90%B3%E5%85%89%E9%90%98%E5%89%AF%E9%99%A2%E9%95%B7(%E5%8F%B3%E4%BA%94)%E3%80%81%E6%96%B0%E7%AB%B9%E7%A7%91%E5%AD%B8%E5%9C%92%E5%8D%80%E7%AE%A1%E7%90%86%E5%B1%80%E8%A8%B1%E5%A2%9E%E5%A6%82%E5%89%AF%E5%B1%80%E9%95%B7(%E5%B7%A6%E5%9B%9B)%E3%80%81%E6%96%B0%E7%AB%B9%E7%B8%A3%E8%A1%9B%E7%94%9F%E5%B1%80%E6%AE%B7%E6%9D%B1%E6%88%90%E5%B1%80%E9%95%B7(%E5%8F%B3%E5%9B%9B)%E3%80%81%E4%B8%AD%E9%86%AB%E5%A4%A7%E6%96%B0%E7%AB%B9%E9%99%84%E9%86%AB%E9%99%B3%E4%BF%8A%E8%B3%A2%E5%89%AF%E9%99%A2%E9%95%B7(%E5%B7%A6%E4%B8%89)%E3%80%81%E5%9C%8B%E7%A0%94%E9%99%A2%E5%84%80%E7%A7%91%E4%B8%AD%E5%BF%83%E6%A5%8A%E7%87%BF%E5%B7%9E%E4%B8%BB%E4%BB%BB(%E5%8F%B3%E4%B8%89)%E3%80%81%E4%B8%AD%E9%86%AB%E5%A4%A7%E6%96%B0%E7%AB%B9%E9%99%84%E9%86%AB%E7%A5%9E%E7%B6%93%E5%A4%96%E7%A7%91%E9%BB%83%E7%A5%A5%E9%8A%98%E4%B8%BB%E4%BB%BB(%E5%B7%A6%E4%BA%8C)%E3%80%81%E5%8F%B0%E7%81%A3%E5%BE%AE%E5%89%B5%E5%85%AC%E5%8F%B8%E6%A2%81%E6%99%83%E5%8D%83%E8%91%A3%E4%BA%8B%E9%95%B7%E3%80%81%E6%96%B0%E7%AB%B9%E7%B8%A3%E8%AD%B0%E6%9C%83%E8%94%A1%E5%BF%97%E7%92%B0%E8%AD%B0%E5%93%A1(%E5%8F%B3%E4%B8%80)%E3%80%81%E6%96%B0%E7%AB%B9%E7%B8%A3%E8%AD%B0%E6%9C%83%E5%90%B3%E6%97%AD%E6%99%BA%E8%AD%B0%E5%93%A1(%E5%B7%A6%E4%B8%80).JPG)
